
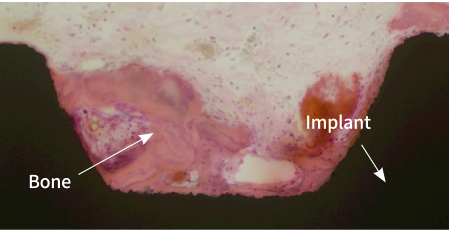
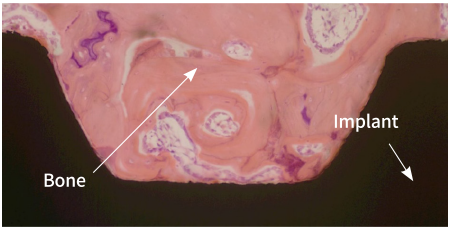
ACTILINK™ motion is a novel vacuum plasma device to removes contaminants such as hydrocarbons which deter enhance osseointegration efficacy of implant fixture.
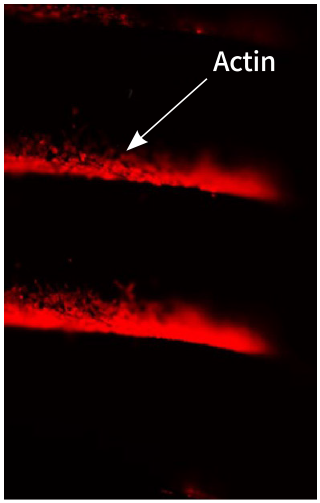
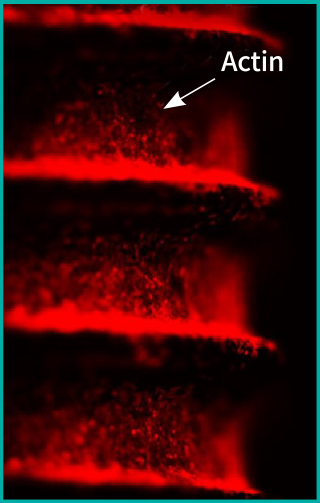
ACTILINK™ has been validated validated on adhesion, proliferation and differentiation of osteoblast cells as well as the adsorption of protein.
ACTILINK™ makes high-performance implant surfaces more perfect.

| Model | ACTILINK™ motion |
|---|---|
| Size (W x D x H) | 168 x 254 x 340mm |
| Weight | 6kg |
| Cycle Time | 60 sec |


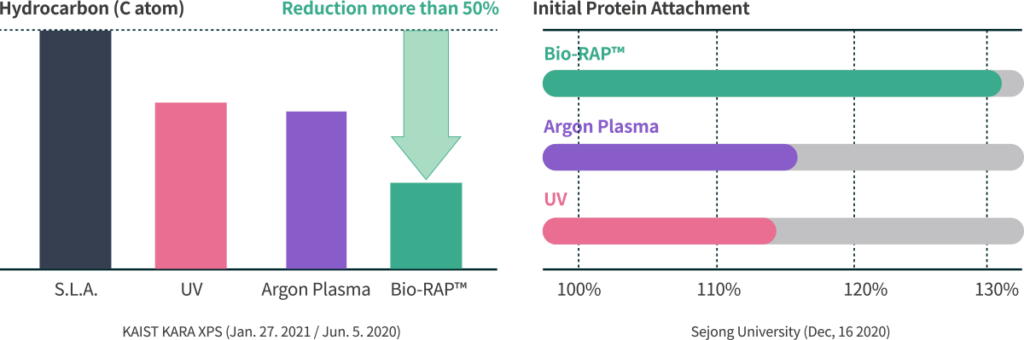
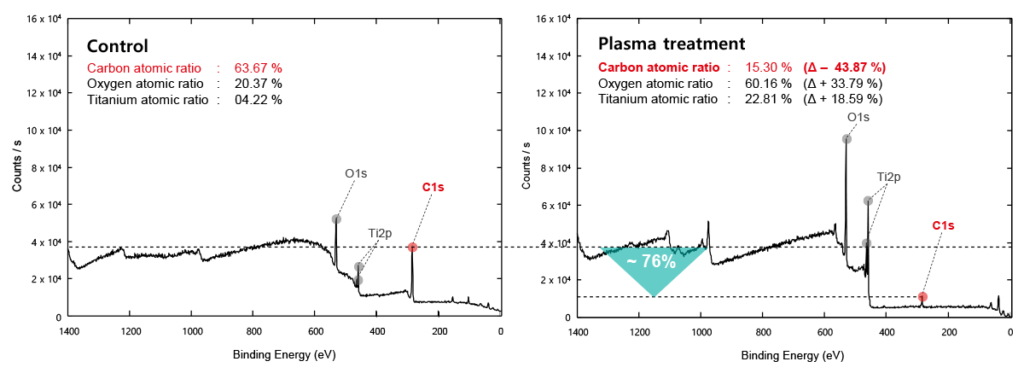

Control
Before the Treatment
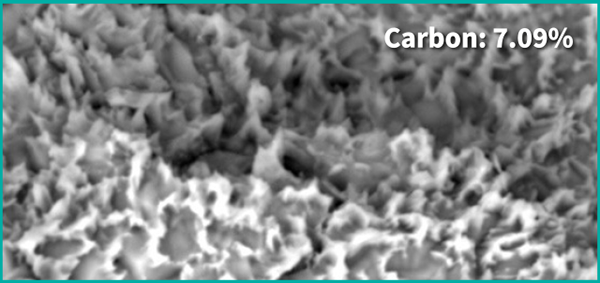

Control
Before the Treatment
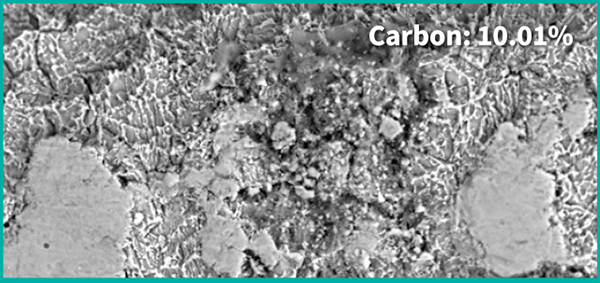
Control
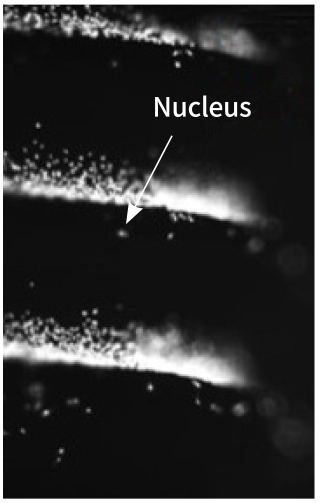
Before the Treatment
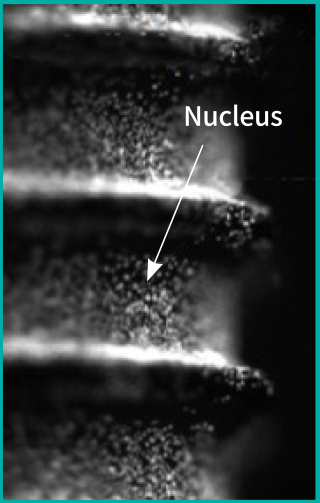
Control
Before the Treatment
Plasmapp Co., Ltd.
Sales & Marketing | 59, Seocho-daero 77-gil, Seocho-gu, Seoul, Republic of Korea [06611]
R&D 1 | 9, Giheungdanji-ro 24beon-gil, Giheung-gu, Yongin-si, Gyeonggi-do, Republic of Korea [17086]
R&D 2 | 372, Dongbu-daero, Osan-si, Gyeonggi-do, Republic of Korea [18151]
Factory | 102, Cheombok-ro, Dong-gu, Daegu, Republic of Korea [41061]
HQ | 125, Gwahak-ro, Yuseong-gu, Daejeon, Republic of Korea [34141]