Low temperature plasma sterilization technology for prevent infection accidents


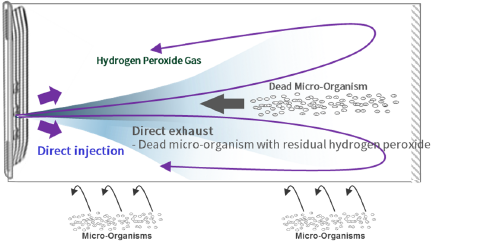
The biological inactivation of Plasmapp is the world’s fastest low-temperature sterilization solution
using sterilant direct injection technology with an impermeable sterile package to maximize sterilant
efficiency. This solution prevents thermal damage to delicate medical devices and secures user safety
in the sterilization process using differentiated plasma technology of Plasmapp.

- 7 minutes fast sterilization with the world's first direct injection pouch and patented sterilization technology

Express Mode (STERLINK™ MINI only) - 12minutes fast sterilization Double cycle sterilization process is applied complying with ISO14937

Vaccum sealing pouches enable staff to have an immediate check on the sterile condition of the sterilized tools inside

Compatible with most medical devices and materials that are heat and moisture intolerable

Safe disposable sterilant cassettes

Simple to operate, install, and monitor (only requires electrical outlet)


Publication expected in 2021 (AIP) - https://doi.org/10.1063/5.0048688
Review of Scientific Instruments 92, 064902 (2021) conducted by Dr. Youbong Lim, Dr. Wonho Choe, Dr. Seung Hun Lee,
Dr. Jun Young Kim and Dr. Hyun Jeong Jeon

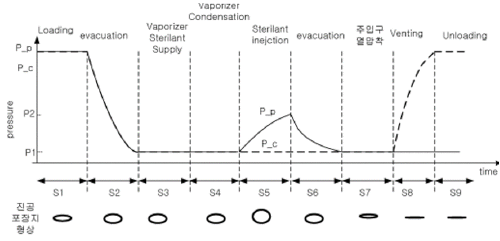
Super-fast sterilizaition cycle (Overall cycle time : 7min)

Reliable sterilization performance (SAL of 10-6: ISO 14937)

Extraordinary performance (SUS Single Lumen: Ø 0.7 mm x 500 mm)

Low temperature reducing maintenance cost of expensive device (Operating temperature < 55℃)

14 FDA certificates including CE (Sales in 52 countries since 2018)
(The first non-US plasma sterilizer clearance by US FDA)
510(K) clearance for STERLINK sterilizer system of Plasmapp
(K212200 / K212193 / K212198)


Global plasma technology company penetrated more than 50 countries

Plasmapp Co., Ltd.
Sales & Marketing | 59, Seocho-daero 77-gil, Seocho-gu, Seoul, Republic of Korea [06611]
R&D 1 | 9, Giheungdanji-ro 24beon-gil, Giheung-gu, Yongin-si, Gyeonggi-do, Republic of Korea [17086]
R&D 2 | 372, Dongbu-daero, Osan-si, Gyeonggi-do, Republic of Korea [18151]
Factory | 102, Cheombok-ro, Dong-gu, Daegu, Republic of Korea [41061]
HQ | 125, Gwahak-ro, Yuseong-gu, Daejeon, Republic of Korea [34141]